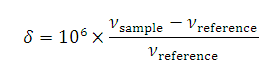The most useful thing about NMR is that the
1H (or
13C) nuclei do not all absorb at the same frequency. The electrons around each nucleus (such as those in bonds and lone pairs) shield the nucleus slightly from the magnetic field supplied by the spectrometer. The actual magnetic field experienced by a nucleus is
smaller than it would have done if the electrons were not there. The
smaller the magnetic field experienced by the nucleus, the
smaller the energy difference between the two alignments of its spin and the
lower the frequency of the radiofrequency radiation that will be absorbed.
The amount of shielding depends on the electron density around each nucleus. Only if the electron density is exactly the same will the shielding be the same.
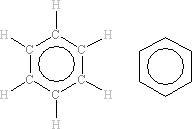
In the benzene molecule, all six hydrogen atoms are in exactly the same environment as are all six carbon atoms. In the NMR spectrum, all six 1H nuclei will absorb at exactly the same frequency as each other. In the 13C NMR spectrum, all six 13C nuclei will similarly absorb at exactly the same frequency as each other.
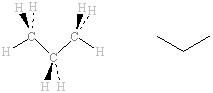
In the propane molecule, the hydrogen atoms on the two CH3 groups at eiher end are in exactly the same environment, as are the two carbon atoms. Again, the six 1H nuclei will absorb at the same frequency as each other. The two 1H nuclei on the central CH2 group are, however, not the same as those on the ends. Although both types of 1H sre bonded to C, the 1H on the ends are bonded to carbon atoms which are bonded to 3 × H and 1 × C whereas the 1H on the central carbon are bonded to a carbon atom which is bonded to 2 × H and 2 × C. The 1H NMR thus shows two signals due to two 1H types. The 13C NMR will also show two signals due to the two 13C types. |
|
 |
Anything that
removes electron density from around a nucleus
deshields it and
shifts the absorption to higher frequency. The more electronegative the atoms that are attached to the nucleus, the higher the absorption frequency.
The Chemical Shift Scale
Unlike
IR and
UV / Visible spectroscopy, it is uncommon to report NMR frequencies as they depend on the strength of the magnetic field and hence on the spectrometer being used. It is more convenient to use a scale that is independent of the spectrometer and its location in the world. To do this, the shift from an agreed reference is measured and this
difference is divided to the absorption frequency of the reference. Since both the absorption frequency of the sample and the reference depend on the strength of the magnetic field in the same way, this 'chemical shift', δ, is independent of the magnetic field.
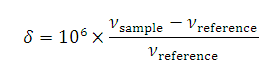
|
νsample and νreference are the absorption frequencies of the sample and reference respectively. These values depend on the size of the magnetic field: the ratio does not.
The factor of 106 leads to values which are easy to remember. Because of this factor, chemical shifts are expressed in 'parts per milion' (ppm).
|
In both
1H NMR and
13C NMR, the same reference compound is used, tetramethylsilane, Si(CH
3)
4, or 'TMS'. In this molecule, all of the
1H nuclei are exactly the same as are all of the
13C nuclei. TMS thus shows only one peak in the
1H NMR and one peak in the
13C NMR. It is also relatively inert.
Characteristic Chemical Shifts
Like
IR spectroscopy, NMR is able to provide information on the types of functional groups present as each has a characteristic chemical shift range. The tables below should be consulted to correlate a chemical shift value with the functional group.